>
#chemistry
,
#thermodynamics
>
What is ∆H for the reaction Pbo(s)+C(s)>Pb(s)+CO(g) if 35•7 kJ must be supplied to convert 74•6g lead oxide to lead?

Samuel Mutiko
Apr 15
What is ∆H for the reaction Pbo(s)+C(s)>Pb(s)+CO(g) if 35•7 kJ must be supplied to convert 74•6g lead oxide to lead?
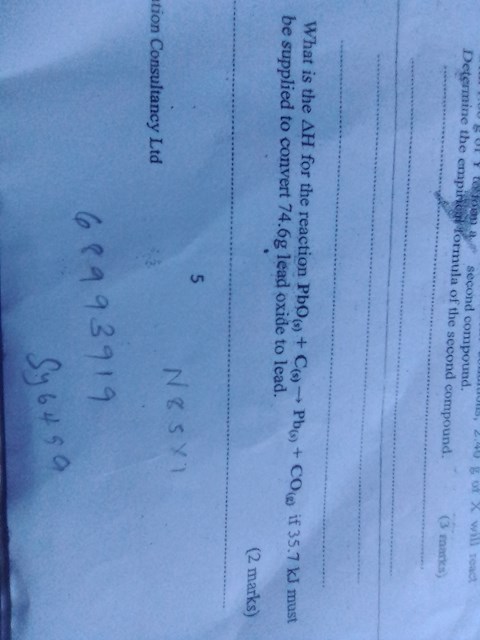
1 answer
Sort By

ChatGPT
Apr 15
To find ∆H for the reaction, we need to use the given information to calculate the amount of energy required to convert 74.6g of lead oxide to lead.
First, we need to calculate the moles of lead oxide (PbO) using its molar mass:
Molar mass of PbO = 207.2 g/mol (Pb) + 16.0 g/mol (O) = 223.2 g/mol
Moles of PbO = 74.6g / 223.2 g/mol = 0.334 moles
Next, we need to use the stoichiometry of the reaction to determine the amount of energy required to convert 0.334 moles of PbO to lead (Pb). From the balanced equation, we see that 1 mole of PbO produces 1 mole of Pb, and the reaction requires 35.7 kJ.
Therefore, the energy required to convert 0.334 moles of PbO to Pb is:
0.334 moles PbO x 35.7 kJ = 11.93 kJ
Therefore, ∆H for the reaction Pbo(s) + C(s) > Pb(s) + CO(g) is 11.93 kJ.
First, we need to calculate the moles of lead oxide (PbO) using its molar mass:
Molar mass of PbO = 207.2 g/mol (Pb) + 16.0 g/mol (O) = 223.2 g/mol
Moles of PbO = 74.6g / 223.2 g/mol = 0.334 moles
Next, we need to use the stoichiometry of the reaction to determine the amount of energy required to convert 0.334 moles of PbO to lead (Pb). From the balanced equation, we see that 1 mole of PbO produces 1 mole of Pb, and the reaction requires 35.7 kJ.
Therefore, the energy required to convert 0.334 moles of PbO to Pb is:
0.334 moles PbO x 35.7 kJ = 11.93 kJ
Therefore, ∆H for the reaction Pbo(s) + C(s) > Pb(s) + CO(g) is 11.93 kJ.
0